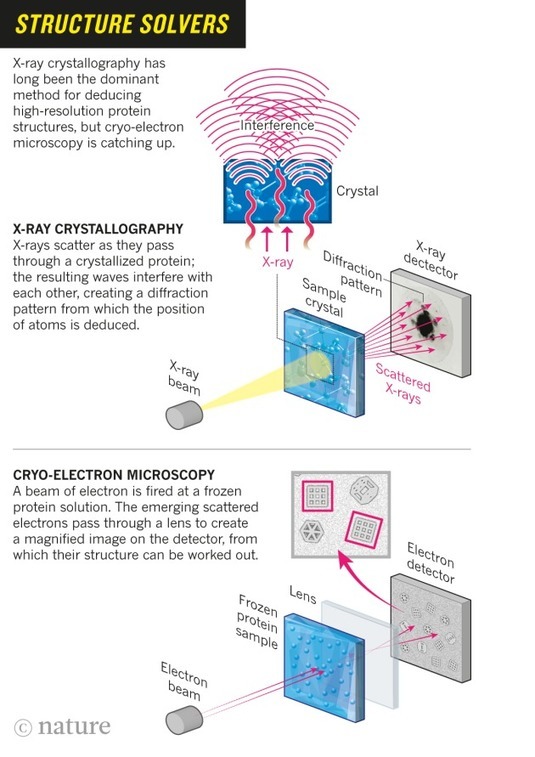
The revolution will not be crystallized: a new method sweeps through structural biology
In a basement room, deep in the bowels of a steel-clad building in Cambridge, a major insurgency is under way.
A hulking metal box, some three meters tall, is quietly beaming terabytes’ worth of data through thick orange cables that disappear off through the ceiling. It is one of the world’s most advanced cryo-electron microscopes: a device that uses electron beams to photograph frozen biological molecules and lay bare their molecular shapes. The microscope is so sensitive that a shout can ruin an experiment, says Sjors Scheres, a structural biologist at the UK Medical Research Council Laboratory of Molecular Biology (LMB), as he stands dwarfed beside the £5-million (US$7.7-million) piece of equipment. “The UK needs many more of these, because there’s going to be a boom,” he predicts.
In labs around the world, cryo-electron microscopes such as this one are sending tremors through the field of structural biology. In the past three years, they have revealed exquisite details of protein-making ribosomes, quivering membrane proteins and other key cell molecules, discoveries that leading journals are publishing at a rapid clip. Structural biologists say — without hyperbole — that their field is in the midst of a revolution: cryo-electron microscopy (cryo-EM) can quickly create high-resolution models of molecules that have resisted X-ray crystallography and other approaches, and labs that won Nobel prizes on the back of earlier techniques are racing to learn this upstart method. The new models reveal precisely how the essential machinery of the cell operates and how molecules involved in disease might be targeted with drugs.
“There’s a huge range of very important biological problems that are now open to being tackled in a way that they could never before,” says David Agard, a structural cell biologist at the University of California, San Francisco.
Scheres was recruited to the LMB several years ago to help push cryo-EM technology to its limits — and he and his colleagues have done just that. Last month, they reported one of the burgeoning field’s most impressive feats: a startlingly clear picture of an enzyme implicated in Alzheimer’s disease, showing the position of its 1,200 or so amino acids down to a resolution of a few tenths of a nanometer1.
Biologists are now pushing the technique further to deduce ever more detailed structures of small and shape-shifting molecules — a challenge even for cryo-EM. “Whether you call it revolution or a quantum leap, the fact is that the gates have opened,” says Eva Nogales, a structural biologist at the University of California, Berkeley.
Sourced through Scoop.it from: www.nature.com
See on Scoop.it – Virology and Bioinformatics from Virology.ca